No products in the cart.
Isopropylphenidate crystal rocks
€11.95
and has been speculated to act as both a dopamine reuptake inhibitor and norepinephrine reuptake inhibitor. This product is intended for forensic and research uses.
Purity: 98.0%
| 1 Gram | €11.95 |
| 3 Grams | €32.95 |
| 5 Grams | €49.95 |
| 10 Grams | €84.95 |
| 25 Grams | €167.95 |
Isopropylphenidate crystal rocks – More information
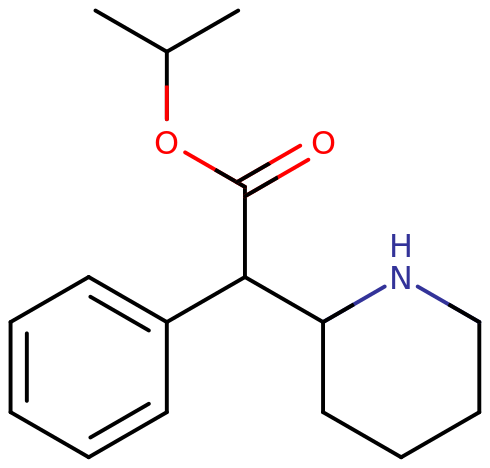
Isopropylphenidate (also known as IPH, IPPH, and IPPD) is synthetic stimulant of the piperidine chemical class that produces stimulating, motivating, and focus enhancing effects when administered. It is a structural analog of the widely-prescribed ADHD medication methylphenidate and is reported to produce near identical cognitive and physical effects, albeit with less of a euphoric “rush” component and a drawn-out duration of action, properties that many find preferable for use as a study-aid or productivity enhancer.
Isopropylphenidate has been investigated for its potential use as a replacement for methylphenidate in the treatment of ADHD and related disorders. One study found that it displayed the same basic activity as a norepinephrine–dopamine reuptake inhibitor (NDRI), possessing, along with both methylphenidate and ethylphenidate, an appreciably high affinity for the dopamine transporter and effects on its cellular reuptake. It displayed comparably minor effects on norepinephrine, however, which was theorized to mean it may possess a more desirable safety and toxicity profile.
Isopropylphenidate has an extremely short history of recreational use in human and has yet to be documented being sold on the streets. It was initially released following the banning of ethylphenidate, which on April 2015 became illegal in the United Kingdom following a temporary-then-permanent blanket ban. Shortly after, it became made available for sale on the online gray market as a research chemical for global distribution.
As of 2017, isopropylphenidate continues to remain available and ambiguously legal in many parts of the world, distributed almost exclusively by online research chemical vendors.
clinical data
| Common names | Isopropylphenidate, IPH, IPPH |
| Substitutive name | Isopropylphenidate |
| Systematic name | Propan-2-yl 2-phenyl-2-(piperidin-2-yl)acetate |
| Psychoactive class | Stimulant |
| Chemical class | Phenidate / Piperidine |
Isopropylphenidate dosage table
| Threshold | 2 – 5 mg |
| Light | 5 – 10 mg |
| Common | 10 – 20 mg |
| Strong | 20 – 35 mg |
| Heavy | 35 mg + |
Isopropylphenidate effect progress
| Total | 2.5 – 4 hours |
| Offset | 1 – 1.5 hours |
Chemistry
Isopropylphenidate is a synthetic molecule of the substituted phenethylamine and piperidine classes. It contains a phenethylamine core featuring a phenyl ring bound to an amino (NH2) group via an ethyl chain. It is structurally similar to amphetamine, featuring a substitution at Rα which is then incorporated into a piperidine ring ending at the terminal amine of the phenethylamine chain. Additionally, it contains an isopropyl acetate bound to R2 of its molecular structure, a noticeable departure from methylphenidate, which contains a methyl group in this position.
Isopropylphenidate structurally diverges from ethylphenidate and methylphenidate by the length of the carbon chain on their acetate group. Iso- regards the side chain of one carbon atom branching into two bound methyl groups, phen- indicates the phenyl ring, id- is contracted from the piperidine ring, and -ate indicates the acetate group. Isopropylphenidate is a chiral compound, and has been documented as being produced as a racemic mixture and exclusively as either of its enantiomers.
Toxicity
The toxicity and long-term health effects of recreational isopropylphenidate use do not seem to have been studied in any scientific context and the exact toxic dosage is unknown. This is because isopropylphenidate has very little history of human usage. Anecdotal evidence from people who have tried isopropylphenidate within the community suggests that there do not seem to be any negative health effects attributed to simply trying this drug at low to moderate doses by itself and using it sparingly (but nothing can be completely guaranteed).
It is strongly recommended that one use harm reduction practices when using this drug.
Tolerance
As with other stimulants, the chronic use of isopropylphenidate can be considered moderately addictive with a high potential for abuse and is capable of causing psychological dependence among certain users. When addiction has developed, cravings and withdrawal effects may occur if a person suddenly stops their usage.
Tolerance to many of the effects of isopropylphenidate develops with prolonged and repeated use. This results in users having to administer increasingly large doses to achieve the same effects. After that, it takes about 3 – 7 days for the tolerance to be reduced to half and 1 – 2 weeks to be back at baseline (in the absence of further consumption). Isopropylphenidate presents cross-tolerance with all dopaminergic stimulants, meaning that after the consumption of isopropylphenidate all stimulants will have a reduced effect.
Psychosis
Abuse of compounds within the stimulant class at high dosages for prolonged periods of time can potentially result in a stimulant psychosis that may present with a variety of symptoms (e.g., paranoia, hallucinations, or delusions). A review on treatment for amphetamine, dextroamphetamine, and methamphetamine abuse-induced psychosis states that about 5–15% of users fail to recover completely. The same review asserts that, based upon at least one trial, antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.
| amount | 1 Gram, 3 Grams, 5 Grams, 10 Grams, 25 Grams |
|---|
Only logged in customers who have purchased this product may leave a review.
Related products
Stimulant
Rated 4.00 out of 5
€13.95Sale!
New
Antipsychotic
Stimulant
€10.95
Entactogens
€11.95
Entactogens
€11.95
Stimulant
€10.95
Stimulant
€11.95
Stimulant
€10.95






















Reviews
There are no reviews yet.