No products in the cart.
Caffeine crystal rocks
€10.95
Caffeine is a methylxanthine alkaloid naturally found in various plant parts that acts as an antagonist at central adenosine receptors at relevant physiological concentrations . It alters fatigue, mood, alertness, motor reaction time, vascular hemodynamics, and pain sensation. Caffeine has also been implicated in carcinogenesis, although the concentrations used to affect cell cycling and apoptosis in the laboratory may not be commonly achieved in vivo.
Purity: 96.5%
| 1 Gram | €10.95 |
| 3 Grams | €27.95 |
| 5 Grams | €39.95 |
| 10 Grams | €74.95 |
| 25 Grams | €159.95 |
Caffeine crystal rocks – More information
For the coffee plant, see Coffea (botany).
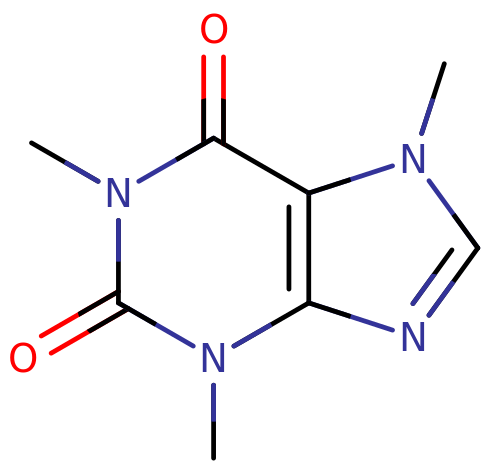
1,3,7-Trimethylxanthine (also known as caffeine) is a naturally-occurring stimulant substance of the xanthine class. Notable effects include stimulation, wakefulness, enhanced focus and motivation. It is the most widely consumed psychoactive substance in the world.
Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants where it acts as a natural pesticide, as well as enhancing the reward memory of pollinators. It is most commonly consumed by humans in infusions extracted from the seed of the coffee plant and the leaves of the tea bush, as well as from various foods and drinks containing products derived from the kola nut.
Unlike many other psychoactive drugs, caffeine is legal and unregulated in nearly all parts of the world. Beverages containing caffeine, such as coffee, tea, soft drinks, and energy drinks, enjoy great popularity. Caffeine is the most commonly used drug in the world, with 90% of adults in North America consuming it on a daily basis. Global consumption of caffeine has been estimated at 120,000 tonnes per year, making it the world’s most popular psychoactive substance. This amounts to one serving of a caffeinated beverage for every person every day.
clinical data
| Common names | Caffeine |
| Substitutive name | 1,3,7-Trimethylxanthine[1] |
| Systematic name | 1,3,7-Trimethylpurine-2,6-dione[2] |
| Psychoactive class | Stimulant |
| Chemical class | Xanthine |
Caffeine dosage table
| Threshold | 2.5 mg |
| Light | 10 – 25 mg |
| Common | 25 – 40 mg |
| Strong | 40 – 80 mg |
| Heavy | 80 mg + |
Caffeine effect progress
| Total | 1 – 2.5 hours |
| After effects | 6 – 24 hours |
Chemistry
Caffeine, or 1,3,7-trimethylpurine-2,6-dione, is an alkaloid with a substituted xanthine core. Xanthine is a substituted purine comprised of two fused rings: a pyrimidine and an imidazole. Pryimidine is a six-membered ring with nitrogen constituents at R1 and R3; imidazole is a 5 membered ring with nitrogen substituents at R1 and R3. Xanthine contains oxygen groups double-bonded to R2 and R6.
Caffeine contains additional methyl substitutions at R1, R3 and R7 of its structure. These are bound to the open nitrogen groups of the xanthine skeleton. It is an achiral aromatic compound.
Metabolites
Caffeine is metabolized in the liver by the cytochrome P450 oxidase enzyme system, in particular, by the CYP1A2 isozyme, into three dimethylxanthines, each of which has its own effects on the body:
Toxicity
Caffeine is not known to cause brain damage, and has an extremely low toxicity relative to dose. There are relatively few physical side effects associated with caffeine exposure. Various studies have shown that in reasonable doses in a careful context, it presents no negative cognitive, psychiatric or toxic physical consequences of any sort.
Lethal dosage
Extreme overdose can result in death. The median lethal dose (LD50) given orally is 192 milligrams per kilogram in rats. The LD50 of caffeine in humans is dependent on individual sensitivity, but is estimated to be about 150 to 200 milligrams per kilogram of body mass or roughly 80 to 100 cups of coffee for an average adult. Though achieving lethal dose of caffeine would be difficult with regular coffee, it is easier to reach high doses with caffeine pills, and the lethal dose can be lower in individuals whose ability to metabolize caffeine is impaired.
It is strongly recommended that one use harm reduction practices when using this substance.
Abuse
Caffeine produces dependence with chronic use and has a low abuse potential. When dependence has developed, cravings and withdrawal effects will occur if one suddenly stops their use.
Tolerance to many of the effects of caffeine develops with prolonged and repeated use. This results in users having to administer increasingly large doses to achieve the same effects. After tolerance has developed, it takes about 3 – 7 days for the tolerance to be reduced to half and 1 – 2 weeks to return to baseline in the absence of further consumption.
Caffeine does not produce cross-tolerance with other stimulants.
Withdrawal symptoms
Withdrawal symptoms – including headaches, irritability, inability to concentrate, drowsiness, insomnia, and pain in the stomach, upper body, and joints –- may appear within 12 to 24 hours after discontinuation of caffeine intake, peak at roughly 48 hours, and usually last from 2 to 9 days. Withdrawal headaches are experienced by 52% of people who stopped consuming caffeine for two days after an average of 235 mg caffeine per day prior to that. In prolonged caffeine drinkers, symptoms such as increased depression and anxiety, nausea, vomiting, physical pains and intense desire for caffeine containing beverages are also reported. Peer knowledge, support and interaction may aid withdrawal.
Psychosis
There is limited evidence that caffeine, in high doses or when chronically abused, may induce psychosis in normal individuals and worsen pre-existing psychosis in those diagnosed with schizophrenia. Caffeine has been shown to potentiate the effects of methamphetamine, which can also induce psychosis.
Legal status
Caffeine is legal in nearly all parts of the world. However, it is often regulated because it is a psychoactive substance. For example, in the United States, the Food and Drug Administration (FDA) restricts beverages to contain less than 0.02% caffeine. unless they are listed as a dietary supplement.
References
| amount | 1 Gram, 3 Grams, 5 Grams, 10 Grams, 25 Grams |
|---|
Only logged in customers who have purchased this product may leave a review.
Related products
Psychedelic
Rated 3.00 out of 5
€11.95Stimulant
Rated 4.00 out of 5
€13.95Entactogens
€11.95
Stimulant
€10.95
Stimulant
€11.95
Stimulant
€10.95
Stimulant
€13.95
Stimulant
€13.95


















Reviews
There are no reviews yet.