No products in the cart.
MDA crystal rocks
€10.95
MDA 19 is a selective agonist of the human peripheral cannabinoid . Surprisingly, it acts as an inverse agonist at the rat CB2 receptor in cell-based functional assays. MDA19 attenuates tactile allodynia produced by spinal nerve ligation or paclitaxel in a dose-related manner in rats and in CB2 mice but not in CB2 mice, indicating that CB2 receptors mediated the effects of MDA19. These effects of MDA 19 are blocked by the selective CB2 antagonist AM630.
Purity: 98.0%
| 1 Gram | €10.95 |
| 3 Grams | €27.95 |
| 5 Grams | €39.95 |
| 10 Grams | €74.95 |
| 25 Grams | €159.95 |
MDA crystal rocks – More information
3,4-Methylenedioxyamphetamine (also known as MDA, and Tenamfetamine, or colloquially as “Sally”, “Sass”, or “Sass-a-frass”) is a synthetic entactogen of the amphetamine chemical class. It produces long-lived entactogenic, stimulant and mild psychedelic effects that include stimulation, anxiety suppression, enhanced feelings of empathy, affection, and sociability, and euphoria when administered.
MDA was first synthesized in 1910 but its psychoactive effects were not discovered until in 1930. It was used in animal and human trials between 1939 and 1941 and from 1949 to 1957. More than 500 human subjects were given MDA in an investigation of its potential use as either an antidepressant or anorectic. By 1958, it was successfully patented as a cough suppresant and ataractic. By 1961 it was patented as an anorectic under the trade name “Amphedoxamine”.
Contemporary reports suggest that MDA emerged as a recreational drug towards the end of 1967, meaning its use predates its more widely used relative MDMA (Ecstasy).
As with MDMA, MDA is thought to act primarily as a serotonin–norepinephrine–dopamine reuptake inhibitor and releasing agent. However, MDA is significantly more potent by weight and subjective intensity relative to MDMA. It also has a notably longer duration (six to eight hours instead of three to five) and produces more traditional serotonergic psychedelic effects (such as visual distortions) along with appreciably higher activity on dopamine, which is also believed to be responsible for the greater degree of neurotoxicity it produces.
Today, possession of MDA is illegal in most countries, although some limited exceptions exist for scientific and medical research.
clinical data
| Common names | MDA, Sass, Sally, Tenamfetamine |
| Substitutive name | 3,4-Methylenedioxyamphetamine |
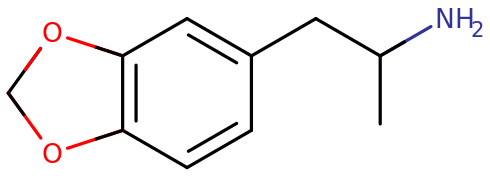
| Systematic name | (R) 1-(benzo[1,3]dioxol-5-yl)propan-2-amine |
| Psychoactive class | Entactogen / Stimulant / Psychedelic |
| Chemical class | Amphetamine / MDxx |
MDA dosage table
| Threshold | 20 – 40 mg |
| Light | 40 – 60 mg |
| Common | 60 – 100 mg |
| Strong | 100 – 145 mg |
| Heavy | 145 mg + |
MDA effect progress
| Total | 5 – 8 hours |
| Offset | 2 – 3 hours |
| After effects | 4 – 48 hours |
History and culture
MDA was originally synthesized by G. Mannish and W. Jacobson in 1910. However, its psychoactive effects were not discovered until the self-experiments of Gordon Alles in July 1930. Alles would later license the drug to Smith, Kline & French. The first animal tests occurred in 1939, followed by human trials in 1941 that explored it as a possible therapy for Parkinson’s disease. From 1949 to 1957, more than 500 human subjects were given MDA in an investigation of its potential use as an antidepressant and anorectic by Smith, Kline & French.
The United States Army also experimented with the drug, code-named EA-1298, while working to develop a truth drug or incapacitating agent. A man named Harold Blauer died in January 1953 after being intravenously injected with 450 mg of the drug.
MDA was eventually patented as a cough suppressant by H. D. Brown in 1958, as an ataractic by Smith, Kline & French in 1960, and as an anorectic under the trade name “Amphedoxamine” in 1961. MDA began to appear on the recreational drug scene around 1967. In early 1968, the Bureau of Drug Abuse Control reported the seizure of over 1.4 kilograms of MDA and 11 kilograms of precursors from a clandestine laboratory in New York.
Several researchers, including Claudio Naranjo and Richard Yensen, have explored utilizing MDA in the field of psychotherapy. In 2010, Matthew Baggott and colleagues studied the ability of MDA to invoke mystical experiences and alter vision in healthy volunteers.
Chemistry
MDA, also known as 3,4-methylenedioxyamphetamine, is a synthetic molecule of the amphetamine family. Molecules of the amphetamine class contain a phenethylamine core featuring a phenyl ring bound to an amino (NH2) group through an ethyl chain with an additional methyl substitution at Rα. MDA also contains substitutions at R3 and R4 of the phenyl ring with oxygen groups. These oxygen groups are incorporated into a methylenedioxy ring through a methylene chain. MDA shares this methylenedioxy ring with MDMA, MDAI and more obscure variants like MDEA or MMDA.
Toxicity
Anecdotal evidence from people within the psychonaut community who have tried MDA suggests that there are no negative health effects attributed to simply trying the drug by itself at low to moderate doses and using it very sparingly (but nothing can be completely guaranteed). Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
Harold Blauer died in January 1953 after being intravenously injected with 450 mg of MDA.
MDA is also known to be more neurotoxic when compared to substances such as MDMA or MDE.
It is strongly recommended that one use harm reduction practices when using this substance.
Tolerance
As with other stimulants, the chronic use of MDA can be considered moderately addictive with a high potential for abuse and is capable of causing psychological dependence among certain users. When addiction has developed, cravings and withdrawal effects may occur if a person suddenly stops their usage.
Tolerance to the psychedelic effects of MDA is built almost immediately after ingestion. However, tolerance to the stimulant and entactogenic effects are built up after repeated and heavy usage in a manner that varies between individuals. After that, it takes about 3 days for the tolerance to be reduced to half and 7 days to be back at baseline (in the absence of further consumption). MDA presents cross-tolerance with all psychedelics and most stimulants, meaning that after the consumption of MDA all psychedelics and some stimulants will have a reduced effect.
Serotonin syndrome risk
Combinations with the following substances can cause dangerously high serotonin levels. Serotonin syndrome requires immediate medical attention and can be fatal if left untreated.
References
| amount | 1 Gram, 3 Grams, 5 Grams, 10 Grams, 25 Grams |
|---|
Only logged in customers who have purchased this product may leave a review.
Related products
Stimulant
€13.95
New
Entactogens
€16.95
Entactogens
€11.95
Stimulant
€10.95
Stimulant
€10.95
Entactogens
€11.95
Stimulant
€13.95
Entactogens
€11.95






















Reviews
There are no reviews yet.